Merck has claimed on Friday (Oct 1) that the world’s first pill to treat the notorious Covid-19 has been developed. The drug, called “molnupiravir”, will be prescribed as 4 capsules twice-daily (8 pills per day) for five days – if the U.S. FDA (Food and Drug Administration) authorizes the drug to patients diagnosed with Covid-19. Merck said it would ask the FDA for the green light.
Developed with its partner, Ridgeback Biotherapeutics, Merck said the medicine showed “compelling results” in Phase-3 clinical trials, claiming that it reduces the risk of hospitalization or death by around 50% for patients with mild or moderate cases of Covid. Merck CEO Robert Davis said – “This is going to change how to manage Covid-19”.
The US pharmaceutical company said an interim analysis showed that 7.3% of patients treated with molnupiravir were hospitalized within 29 days. Of the patients who received a placebo, 14.1% were hospitalized or died by day 29. No deaths were reported in patients who were given molnupiravir within the 29-day period, while eight deaths were reported in placebo-treated patients.

The study was conducted on 775 adults with mild to moderate Covid, who were considered higher risk for severe disease owing to health problems such as obesity, diabetes or heart disease. More importantly, Merck said, the drug demonstrated “consistent efficacy” across multiple Coronavirus variants, including the highly transmissible Delta variant.
The Phase-3 trial was conducted at more than 170 sites in the U.S., Brazil, Italy, Japan, South Africa, Taiwan and Guatemala. But Merck is also testing molnupiravir in a separate global Phase-3 study to evaluate its efficacy in preventing the spread of Covid within households. Merck’s stock price shot up more than 9% in early trading after the news.
Ridgeback Biotherapeutics CEO Wendy Holman said – “We are very encouraged by the results from the interim analysis. With the virus continuing to circulate widely, and because therapeutic options currently available are infused or require access to a healthcare facility, antiviral treatments that can be taken at home to keep people with Covid-19 out of the hospital are critically needed.”
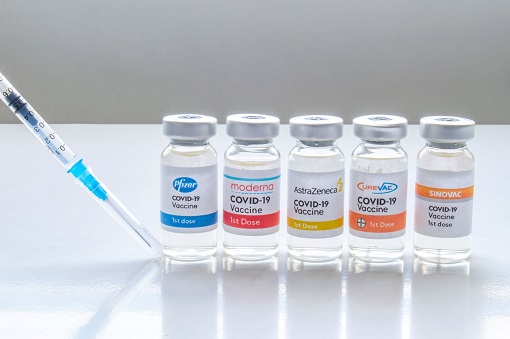
An oral antiviral would indeed be a game changer for obvious reason. Existing treatment of administering Covid-19 vaccines through jabs is tedious and logistically challenging. A simple pill that you can pop into your mouth would be more acceptable to certain people who currently may be terrified to get vaccinated. It’s also less painful to take the Coronavirus vaccine in the form of pills.
Pfizer and Swiss drugmaker Roche Holding AG are also racing to develop a similar easy-to-administer antiviral pill for Covid-19. However, only antibody cocktails which have to be given intravenously are approved for non-hospitalized patients for the time being. The strong performance of Merck share price suggests that the new game is in oral antiviral solution.
In fact, Merck’s drug has seen its spillover effect to other companies that are developing a similar Covid-19 treatment. Atea Pharmaceuticals Inc saw its shares up 20% after the news. Not only pills can be help curb outbreaks in poorer and more remote corners of the world, it will also solve the biggest problem in many countries – shortage of hospital beds for Covid patients in various stages.

The pharmaceutical giant said it expects to produce 10 million courses of the treatment by the end of 2021, and more doses in 2022. The company has a U.S. government contract to supply 1.7 million courses of molnupiravir at a price of US$700 per patient. Merck said it plans to implement a “tiered pricing” approach based on World Bank country income criteria to ensure accessibility.
Merck, which made US$48 billion last year and has 74,000 employees, has also agreed to license the drug to several India-based generic drugmakers, which would be able to supply the treatment to low-and-middle-income countries. Still, it has to wait for the FDA’s approval. Interestingly, it has not said which group of patients it plans to ask the FDA to authorize the drug for.
Technically, Merck’s drug, if authorized, would be the second antiviral treatment for Covid. The first was remdesivir, the same drug trumpeted by Donald Trump when he developed Covid-19 last year. However, not only the WHO has rejected remdesivir, it has also lost favour among clinicians as studies have suggested that it offers only modest benefit for Coronavirus patients.

While Merck offers limited information on side-effects, there’s a catch with molnupiravir. The drug works by creating errors in the virus’s RNA, a tactic that screws up the Coronavirus’ ability to replicate. But it also means the drug is only effective if given in the early stage of infection when the virus is still multiplying in the body. In the trial, it was given within the first five days of symptoms.
Therefore, the molnupiravir pill is not a vaccine, let alone the Holy Grail in fixing the pandemic. It is an oral antiviral, another tool or weapon to fight Covid-19. Vaccinations through jabs are still required to prevent from getting the virus in the first place. The pill provides a good tool for people who refuse to receive vaccine, but ready to take the oral antiviral during the early days of symptoms.
In the U.S., the availability of vaccines for kids soon, hesitancy remains among some parents about inoculating children 5 to 11. While one-third of parents of 5-to-11-year-olds say that they will vaccinate their child as soon as possible, 32% of parents say that they will wait and see how the vaccine is working. A whopping 24% say that they definitely won’t get their kids vaccinated.

Other Articles That May Interest You …
- Mix & Match – CanSino Booster Shot After Second Dose Of Sinovac Covid Vaccine Can Boost Antibodies 78-Fold
- Forget The Third Dose – Get Ready For A 4th Dose Of Covid-19 “Modified” Vaccines As Pandemic Becomes Endemic
- No Evidence – Biden Not Happy After All U.S. Spy Agencies Still “Inconclusive” Over Origins Of Covid-19
- The Third Dose – Get Ready For Covid Vaccine Booster Shot At 8 Months After Second Dose
- Moderna Vaccine Has Similar Side Effects Like Pfizer – But May Be Better Than Pfizer Against Delta Variant
- China Introduces Single-Dose Vaccine – After Broke Record With More Than 15 Million People Vaccinated In A Day
- EU Finally Admits AstraZeneca Covid-19 Vaccine Can Cause Blood Clots – And UK Recommends Get Other Vaccines
- Sinopharm And Sinovac – Should You Trust Any Of These Chinese Covid-19 Vaccines?
- Forget UK’s Covid-19 Variant – This South African Coronavirus Variant Is Deadlier And Can Spread Even Faster
- Coronavirus – Here’s How China Is Back To Normal While The U.S. And Europe Are Getting From Bad To Worse
- 3 Coronavirus Variants Discovered – Surprisingly, “Type-A” Found In Americans, Wuhan’s Type-B And Type-C In Europe

|
|
October 1st, 2021 by financetwitter
|

|

|

|

|

|

|



























Comments
Add your comment now.
Leave a Reply