South Africa has recorded almost 1.5 million Coronavirus cases and more than 46,000 deaths since the pandemic began. And it has bought 1.5 million doses of vaccine from British pharmaceutical AstraZeneca for healthcare workers. The vaccination is scheduled to start this week, when the country suddenly decided to suspend the rollout. What has happened?
Apparently the vaccine, manufactured by AstraZeneca Plc and developed at the University of Oxford, has shown a “disappointing” results against a new Covid-19 variant in South Africa. In a trial conducted in about 2000 people, the efficacy was so low that even against mild and moderate cases, it recorded just 21.9% efficiency – well below minimal international standards.
If the vaccine could not even protect South Africans in the low-risk group, it would be a waste of time to vaccinate people in the high-risk category. The trial participants were 2,000 healthy, young people with an average age of 31. Essentially, the South African variant – also known as 501.V2 or B.1.351 – could still spread from person to person even after vaccination.
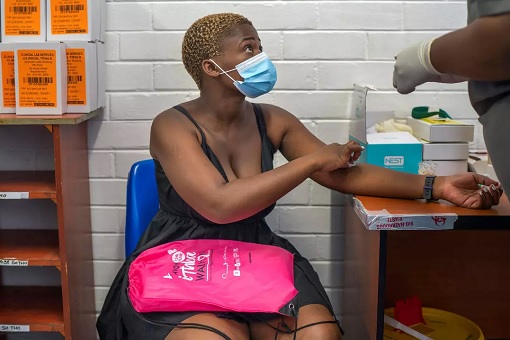
The latest highly contagious variant has spread so widely that 90% of new infections in South Africa are of the new variant. Before the variant began to spread, people who received one dose of the Oxford- AstraZeneca vaccine had shown 75% efficacy against mild and moderate cases. But researchers have found a strong link between the vaccine failure and B.1.351’s explosion.
After the decision by South Africa to halt the use of the vaccine was leaked, Sarah Gilbert, professor of vaccinology at the University of Oxford, which developed the vaccine with AstraZeneca, said that “efforts are underway to develop a new generation of vaccines that will allow protection to be redirected to emerging variants as booster jabs, if it turns out that it is necessary to do so.”
Covid-19 vaccines made by Johnson & Johnson (J&J) and Novavax have also been shown to offer weaker protection against the mutated B.1.351 variant. However, the vaccine efficacy against mild disease in South Africa was 57% for J&J and 49% for Novavax – obviously higher than AstraZeneca’s. With Oxford- AstraZeneca vaccine scrapped, what is the next solution?

Now, South Africa plans to give Johnson & Johnson vaccine to its 1.25-million front-line health workers, even though the vaccine is still being tested internationally and has not been approved in any country. That’s because in other clinical tests in the country, J&J vaccine convincingly protected against severe disease and death – even against the B.1.351 variant.
In a 44,000-person trial, the J&J vaccine prevented 85% of severe cases and completely protected people from hospitalization and death in several countries, including the 15% of South African participants. Both Johnson & Johnson and AstraZeneca vaccines are based on a similar technology – inducing the body to make the spike protein of SARS-CoV-2 by delivering its genes in a harmless adenovirus.
But unlike AstraZeneca, Johnson & Johnson is a single-dose vaccine. J&J is not the only vaccine that the country will use to vaccinate an estimated 40 million people by the end of the year. The country will also be using vaccine manufactured by Pfizer, as well as the Russian Sputnik V, Chinese Sinopharm and Moderna vaccines, according to Health Minister Zweli Mkhize.

Prior to the suspension of AstraZeneca, the country had already ordered 9-million doses of Johnson & Johnson vaccine, which is made by Belgian pharmaceutical firm Janssen, in addition to 20-million jabs from Pfizer. South Africa is in advanced stages of evaluating Russia’s Sputnik V vaccine and is negotiating over an offer made by China for access to its Sinopharm vaccine.
What will happen to the stock of 1.5-million doses of “ineffective” AstraZeneca vaccine? South Africa plans to give the AstraZeneca jab to a group of 100,000 older nurses and healthcare workers in an attempt (or rather an experiment) to see if the vaccine is effective against the new variant and in preventing severe illness in an older age group.
As to the remaining doses of AstraZeneca vaccine, produced in large quantities by the Serum Institute of India, the country may swap or sell it to buyers desperate for the vaccines. Health Minister Zweli Mkhize has claimed that there are ready buyers. But there is a small problem. The AstraZeneca doses that arrived on Feb 1 in South Africa came with an April 30 expiration date.

The early disappointing results of the AstraZeneca vaccine against the South Africa variant could have far-reaching implications. Many other African and poor countries have been planning to use the AstraZeneca vaccine as it is cheaper and does not require storage in ultra-cold freezers.
Other Articles That May Interest You …
- Identify, Investigate, Negotiate – As Malaysia Struggles, Neighbour Singapore Reveals How It Speedily Gained Covid-19 Vaccine
- Before You Rush For Covid-19 Vaccine, Remember This – You Can’t Sue Anyone If Suffer Any Serious Side Effects
- The FDA Says Pfizer Vaccine Is Safe & Effective – But There’re Many Horror Stories, Including Allergy Warning
- Forget UK’s Covid-19 Variant – This South African Coronavirus Variant Is Deadlier And Can Spread Even Faster
- Mistake Or Cheating? – AstraZeneca-Oxford Admits Manufacturing Error In Its Vaccine Effectiveness Results
- Moderna Reveals Covid Vaccine – Here’s How This 94.5% Effective Vaccine Differs From Pfizer’s Vaccine
- Coronavirus – Here’s How China Is Back To Normal While The U.S. And Europe Are Getting From Bad To Worse
- Russia Reveals “Sputnik V” – But Scientists Condemn The Coronavirus Vaccine As Risky, Dangerous & Could Backfire
- 3 Coronavirus Variants Discovered – Surprisingly, “Type-A” Found In Americans, Wuhan’s Type-B And Type-C In Europe
- The World Is Working On 20 Coronavirus Vaccines – But It Could Take Up To 18 Months
- China Appears To Be Winning The Coronavirus War, And Other Countries Are Studying How The Chinese Did It

|
|
February 10th, 2021 by financetwitter
|

|

|

|

|

|

|



























Comments
Add your comment now.
Leave a Reply