Is vaccine developed by Pfizer and German partner BioNTech safe and effective as claimed? According to the U.S. FDA (Food and Drug Administration), their analysis found the vaccine to be 95% effective more than 7 days after the second dose. Between 2 to 7 days after the second dose, the efficacy is 90.5%. The first dose only shows about 52% effectiveness.
Interestingly, the effectiveness varies slightly according to ethnicity, race and age. The white people show 95% while black people demonstrate 100% effectiveness. Meanwhile, American Indian, Asians and Pacific Islanders show 89% efficacy. The vaccine has 94% efficacy in people over 55 years old. Between 16 to 55 years old, the effectiveness is 95%.
However, according to the 53-page public document (downloadable here) containing analysis of the FDA and Pfizer, trial participants demonstrated a truckload of side effects. Symptoms included fever, chills, cough, diarrhea, vomiting, sore throat, fatigue, headache, runny nose or nasal congestion, nausea, muscle pain, shortness of breath and loss of taste or smell.

A staggering 84.1% of trial subjects reported reactions after being injected with Pfizer- BioNTech vaccine. The trial report says 62.9% of participants reported fatigue, headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%) and fever (14.2%). Bell’s palsy – temporary muscle paralysis in the face – was found in four patients who received the vaccine.
A total of 6 deaths occurred in the reporting period (2 deaths in the vaccine group, 4 in placebo). In the vaccine group, one participant with baseline obesity and pre-existing atherosclerosis died 3 days after dose-1, and the other participant experienced cardiac arrest 60 days after dose-2 and died 3 days later. The Pfizer vaccine requires two doses, three weeks apart.
But in reality, two British people with severe allergies reportedly had allergic reactions to Pfizer- BioNTech Covid-19 vaccine, raising questions about whether it is safe for people with pre-existing allergies after the U.K. rolled out the vaccine on Tuesday (Dec 8). The analysis report revealed by the FDA, however, contains very little information about the risks to be faced by people with allergies.

The workaround, as subscribed by the British regulators, is for people with severe allergies to avoid the vaccine, for now. However, this also means the Phase 3 clinical trials conducted by the pharmaceutical companies were not comprehensive enough. According to Moncef Slaoui, co-head of Operation Warp Speed, subjects who have had severe allergic reactions were deliberately excluded in trials.
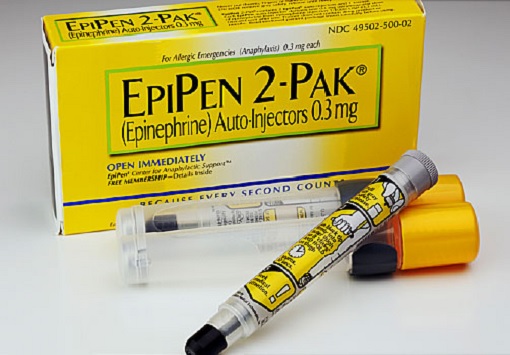
Dr. Peter Hotez, a paediatrician and dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said a vaccine that triggers dangerous reactions in people with severe allergies poses a major challenge in the U.S. About 4 million Americans carry epinephrine (adrenaline) with them at all times in case of allergic reactions to insect stings/bites, foods, drugs, or other substances.
Hotez, confused by the allergic reaction, said – “If you start issuing recommendations that anyone with an EpiPen doesn’t get vaccinated, that could be a showstopper for Americans. It’s very inconsistent. That’s why it’s really puzzling me.” In a statement Wednesday, Pfizer admitted that its trials did not include people with severe allergic reaction.
One participant in Pfizer’s trial told CNBC that he was up all night after the first shot from the pain of the injection. The booster injection he received caused more of that same pain in his arm, followed by intense flu-like symptoms. He couldn’t sleep that night without an electric blanket, and shook so hard that it became uncontrollable and he cracked his tooth.
According to Dr. Greg Vanichkachorn, a medicine specialist of the Mayo Clinic, some Covid-19 patients may experience lingering symptoms for more than a year. He said long-hauler SARS patients during the 2003 epidemic often took more than one year to fully recover. Therefore, he thinks a similar recovery trajectory for post-Covid syndrome patients are absolutely possible.
A week ago, Britain became the first western country to authorize the use of Covid-19 vaccine developed by Pfizer-BioNTech. The approval granted by the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA) on December 1 was at lightning speed – just 3 weeks after Pfizer published the first data from its Phase-3 clinical trial.

European Union regulators – the European Medicines Agency (EMA) – have criticized the U.K. for rushing its approval of Pfizer’s vaccine. EMA, which is in charge of approving Covid-19 vaccines for the EU, said it would only decide by Dec 29 whether to authorise Pfizer’s vaccine. The U.S. FDA, on the other hand, is not scheduled to consider the Pfizer vaccine until Dec 10.
The FDA authorization of the vaccine could come within days after Thursday’s meeting (Dec 10). But ahead of a possible approval in the United States, Canada’s health regulator has approved Pfizer’s Coronavirus vaccine on Wednesday (Dec 9) – the third country to do so after the United Kingdom and Bahrain. Canada is to receive 249,000 doses this month of Pfizer vaccine.
Interestingly, Moderna did not respond to a request for comments about whether people with severe allergies were included in their trials. Both Pfizer and Moderna’s vaccines are built based on genetic material called “mRNA” or messenger RNA vaccines – a new technology that’s never before been licensed in the U.S. These vaccines are not like a normal flu vaccine.
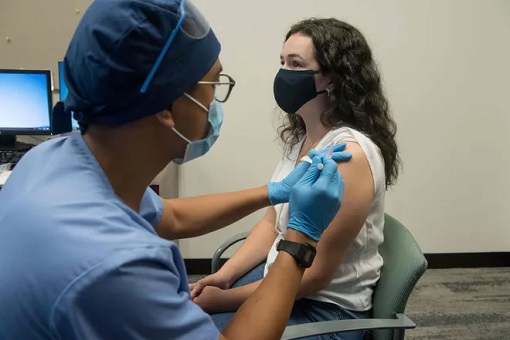
The vaccine introduces the mRNA to the human body, triggering human cells to produce a “spike proteins”. As a result of these proteins, the human body produces antibodies to guard against Coronavirus infection – creating an immune system. The benefit of using mRNA technology is the speed with which it can be manufactured, unlike a traditional vaccine.
Still, all the vaccines developed by Pfizer-BioNTech, Moderna and AstraZeneca-Oxford have something in common – it’s unknown how long the protection will last. Pfizer said it plans to monitor patients for two years to learn more about the protection duration. They also do not know whether the vaccines can prevent asymptomatic infections as well as illness caused by the virus.
Other Articles That May Interest You …
- Here’s Why You Should Not Rush To Get A Covid-19 Vaccine – Even After Britain Approved Pfizer Vaccine
- Mistake Or Cheating? – AstraZeneca-Oxford Admits Manufacturing Error In Its Vaccine Effectiveness Results
- AstraZeneca-Oxford Vaccine Shows 62% To 90% Effectiveness – Here’s Why This Could Be The Best Bet For Everyone
- Moderna Reveals Covid Vaccine – Here’s How This 94.5% Effective Vaccine Differs From Pfizer’s Vaccine
- Covid-19 Vaccines Hit Roadblocks – In 24 Hours, Eli Lilly And Johnson & Johnson’s Trials Suspended Over Safety Concerns
- Coronavirus – Here’s How China Is Back To Normal While The U.S. And Europe Are Getting From Bad To Worse
- Russia Reveals “Sputnik V” – But Scientists Condemn The Coronavirus Vaccine As Risky, Dangerous & Could Backfire
- 3 Coronavirus Variants Discovered – Surprisingly, “Type-A” Found In Americans, Wuhan’s Type-B And Type-C In Europe
- USA vs Europe – The War For Face Masks Get Ugly As The U.S. Accused Of Piracy & Paying 3 Times Cash
- The World Is Working On 20 Coronavirus Vaccines – But It Could Take Up To 18 Months
- China Appears To Be Winning The Coronavirus War, And Other Countries Are Studying How The Chinese Did It
- Arabs Conspiracy Theories – Coronavirus Is The U.S. & Israel Biological Warfare To Cripple China’s Economy & Reputation

|
|
December 10th, 2020 by financetwitter
|

|

|

|

|

|

|
Comments
The “horror stories” will definitely make our kiasu and kiasi types get nice nightmares and lose sleep.
For many they would develop acute anxiety and some would die from the sheer fear of death.
None of the above all that bad. Considering hanging around and watching our rubbish comical gomen (means ALL our politicians) fcuk it all up and kill us all, anyway…



























I am disappointed in how the medical world tackle Covid19 ..
after almost a year, I am still in search on information .. my shallow knowledge and logic come from simple analogy on how we catch a common flu .. as the drug makers already know, a certain dosage is needed for the vaccine to be effective, isn’t it the same we need certain amount of exposure to be infected .. what is the amount, is begging the answer.
Can someone come out with a mask that can indicate virus exposure and at pre-critical infectious level through colouring?