Following Coronavirus vaccines revealed by Pfizer-BioNTech and Moderna after encouraging late-stage trial results, British pharmaceutical giant AstraZeneca announced on Monday that it too has developed a Covid-19 vaccine. Unlike Pfizer and Moderna’s effectiveness of 95% and 94.5%, respectively, AstraZeneca’s vaccine has an average efficacy of 70%.
However, depending on dosage, the latest vaccine’s effectiveness could be as low as 62% and as high as 90%. Apparently, AstraZeneca said its vaccine, developed in collaboration with the University of Oxford, was assessed over two different dosing regimens. No hospitalizations or severe cases of the disease were reported in participants receiving the vaccine during the trials.
One regimen, given to nearly 9,000 people, showed 62% efficacy when given as two full doses at least one month apart. Another regimen showed an effectiveness of 90% when 2,700 trial participants received a half dose, followed by a full dose at least one month apart. Hence, when combined, the analysis from both different dosage regimens produced an average effectiveness of 70%.

However, a vaccine is only as good as its ability to reach the mass market, not to mention its affordability. Therefore, despite the lower efficiency of the British vaccine, it offers distribution and logistic advantages over Pfizer and Moderna’s. While vaccines developed by Pfizer-BioNTech and Moderna have to be stored frozen, Astra-Oxford’s can be kept at refrigerator temperature.
AstraZeneca claims its vaccine can be stored, transported, and handled at normal refrigerated conditions (36º-46 º Fahrenheit or 2º-8º Celsius) for at least six months and administered within existing healthcare configurations. That makes the vaccine easier to transport and store globally, particularly in lower and middle-income countries.
In comparison, Pfizer’s vaccine needs specialised freezers as it must be stored at about minus 70º Celsius (-94º Fahrenheit). It means storage and distribution would be a challenge for some countries, let alone people living in rural areas. If the vaccine is not kept in the super-cold freezers, which cost around US$10,000 a pop, the mRNA can break down, making the vaccine unusable.

Moderna’s Covid-19 vaccine, on the other hand, remains stable at 36º to 46º Fahrenheit (2º to 8º Celsius), the temperature of a standard medical refrigerator – for up to 30 days (as compared to AstraZeneca’s 6 months). If stored at negative 4º Fahrenheit (-15.5º Celsius), the vaccine can be stored up to 6 months. At room temperature, the vaccine is stable for up to 12 hours.
In terms of pricing, Pfizer is reportedly charging about US$20 per dose for its vaccine. Under a special European Union deal for 200 million doses with an option for an additional 100 million doses, the bloc of 27 European countries will pay less than US$19.50 per jab. The U.S. agreed to pay a higher price – more than US$19.50 per injection – for its 100 million doses.
Moderna’s vaccine is more expensive. In August, the U.S. biotechnology firm said it was charging between US$32 and US$37 per dose for its vaccine for some customers – even under the so-called “cheaper pandemic pricing”. Two days ago, Moderna said it will charge governments between US$25 and US$37 per dose, depending on the amount ordered.

According to Financial Times, the AstraZeneca’s vaccine could be priced at US$3 to US$4, obviously much lower than Pfizer and Moderna. The extremely low price is partly due to the company’s “no-profit pledge”, and largely because the Oxford technology is more established – enabling the vaccine to be mass produced easier and cheaper.
Cambridge-based AstraZeneca and University of Oxford are one of the fastest-moving vaccine developers from the early stage of Covid-19 pandemic. After gaining years of experience working on a vaccine against Covid’s relation MERS (Middle East Respiratory Syndrome), Oxford’s scientists had an advantage that allowed them to move quickly to create a jab.
Pascal Soriot, CEO of AstraZeneca, said – “This vaccine’s efficacy and safety confirm that it will be highly effective against Covid-19. Furthermore, the vaccine’s simple supply chain and our no-profit pledge and commitment to broad, equitable and timely access means it will be affordable and globally available, supplying hundreds of millions of doses on approval.”

Both Pfizer-BioNTech and Moderna’s vaccines based on genetic material called “mRNA”. The vaccine introduces the mRNA to the human body, triggering human cells to produce a “spike proteins”. As a result of these proteins, the human body produces antibodies to guard against Coronavirus infection – creating an immune system.
AstraZeneca and Oxford, however, are using a different methodology – a weakened virus that causes the common cold in chimpanzees. First, the chimp virus is modified so it cannot multiply and cause disease in the body. Then, it is uploaded with the gene for the Coronavirus spike protein, the club-shaped part that dots the surface of the virus and is used to penetrate human cells.
When the vaccine is injected, the chimp virus delivers the Coronavirus gene to human cells, which start to produce spike protein. These proteins, when detected by the human body immune system, will produce antibodies that are ready to attack the real Covid-19 virus. Oxford approach is considered safer as it has been used in vaccines for people with diseases like TB, MERS, Ebola and malaria.
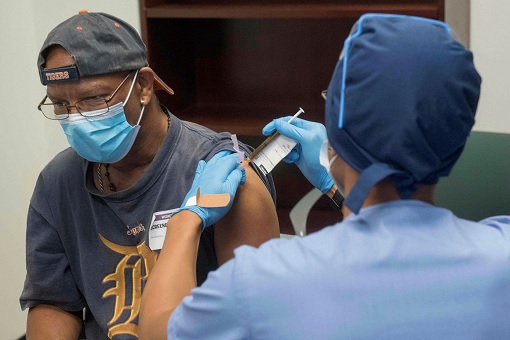
Even though AstraZeneca has produced a third Western-developed shot, Prof. Sarah Gilbert, one of Oxford’s vaccine scientists on the vaccine, said researchers are still trying to figure out why the half-dose regimen has shown to be more effective than a stronger dose. One of the possibilities could be that the lower first dose better mimics the natural human immune system.
The British government has pre-ordered 100 million doses of the AstraZeneca-Oxford vaccine, and AstraZeneca says it will make 3 billion doses for the world next year. Under Europe’s inclusive vaccines alliance, up to 400 million doses of the Oxford vaccine will be made available to European nations, beginning in 2020 (U.K. already has 4 million doses with 96 million to come).
However, the vaccine needs to be approved by regulators to ensure safety, quality and effectiveness. AstraZeneca said it would immediately prepare the regulatory submission of the data to health authorities around the world. Prime Minister Boris Johnson welcomed the “incredibly exciting news” and said that while there were still safety checks to come, “these are fantastic results”.

Still, all the vaccines developed by Pfizer-BioNTech, Moderna and AstraZeneca-Oxford have something in common – it’s unknown how long the protection will last. They also do not know whether the vaccines can prevent asymptomatic infections as well as illness caused by the virus. U.K. and European Union regulators are conducting accelerated reviews of the results from both Astra and Pfizer.
Of the three vaccines, Oxford-developed vaccine appears to be the best bet for the worldwide community, at least for now. The Oxford-AstraZeneca vaccine doesn’t need to be stored in subzero temperatures, making it easier for its global manufacturing, storage and distribution across the world. Besides cheaper, it’s also based on proven methodology and technology.
Other Articles That May Interest You …
- Moderna Reveals Covid Vaccine – Here’s How This 94.5% Effective Vaccine Differs From Pfizer’s Vaccine
- Covid-19 Vaccines Hit Roadblocks – In 24 Hours, Eli Lilly And Johnson & Johnson’s Trials Suspended Over Safety Concerns
- Desperate Trump Orders “Emergency Plasma Treatment” For Covid-19, May Fast-Track Vaccine Like Russia
- Russia Reveals “Sputnik V” – But Scientists Condemn The Coronavirus Vaccine As Risky, Dangerous & Could Backfire
- How Vietnam Won The Coronavirus War And To Emerge The Biggest Winner In Economic Growth In Southeast Asia
- Lawsuits For Trillions Of Dollars Against China Over Spread Of Coronavirus – Here’s Why It’s A Waste Of Time
- 3 Coronavirus Variants Discovered – Surprisingly, “Type-A” Found In Americans, Wuhan’s Type-B And Type-C In Europe
- The World Is Working On 20 Coronavirus Vaccines – But It Could Take Up To 18 Months
- China Appears To Be Winning The Coronavirus War, And Other Countries Are Studying How The Chinese Did It
- Arabs Conspiracy Theories – Coronavirus Is The U.S. & Israel Biological Warfare To Cripple China’s Economy & Reputation

|
|
November 23rd, 2020 by financetwitter
|

|

|

|

|

|

|



























“Still, all the vaccines developed by Pfizer-BioNTech, Moderna and AstraZeneca-Oxford have something in common – it’s unknown how long the protection will last. They also do not know whether the vaccines can prevent asymptomatic infections as well as illness caused by the virus.”
Means in actual fact, they are a pile of bollocks!
Not enough is known of the tests, not enough tests, no long-term results as yet available, the protocols read wishy-washy, speed of development open to suspicion, shed loads of hyper hype.
If anything (for a small minority) they are more temporary symptom-relieving than cure or of help to prevent re-infection. Or stopping infection between people.
Nothing to wet oneself over.
Go back to sleep.